Lothar Meyers Classification In The Form Of Curve
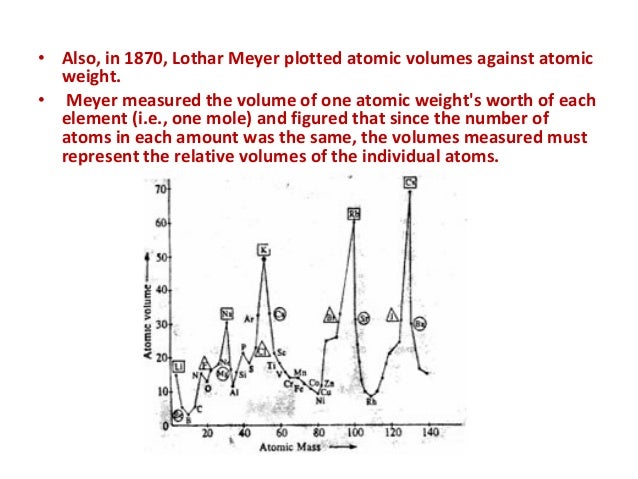
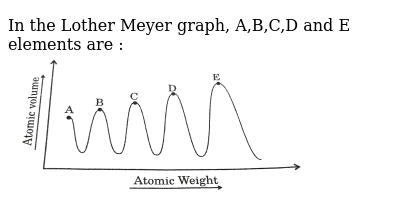
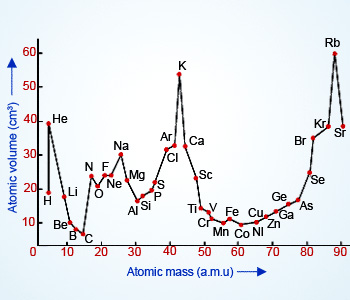
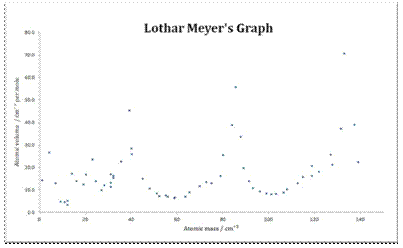
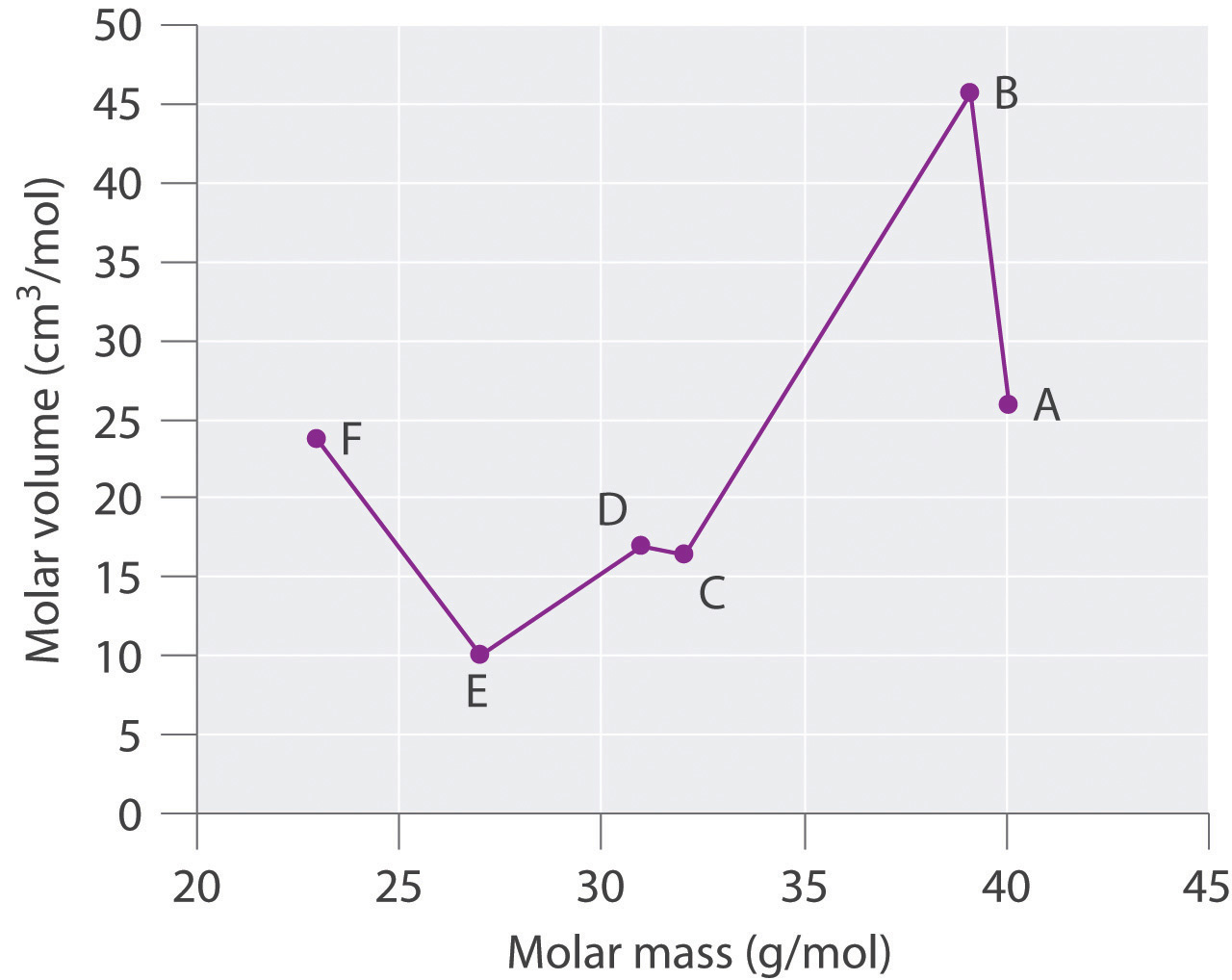
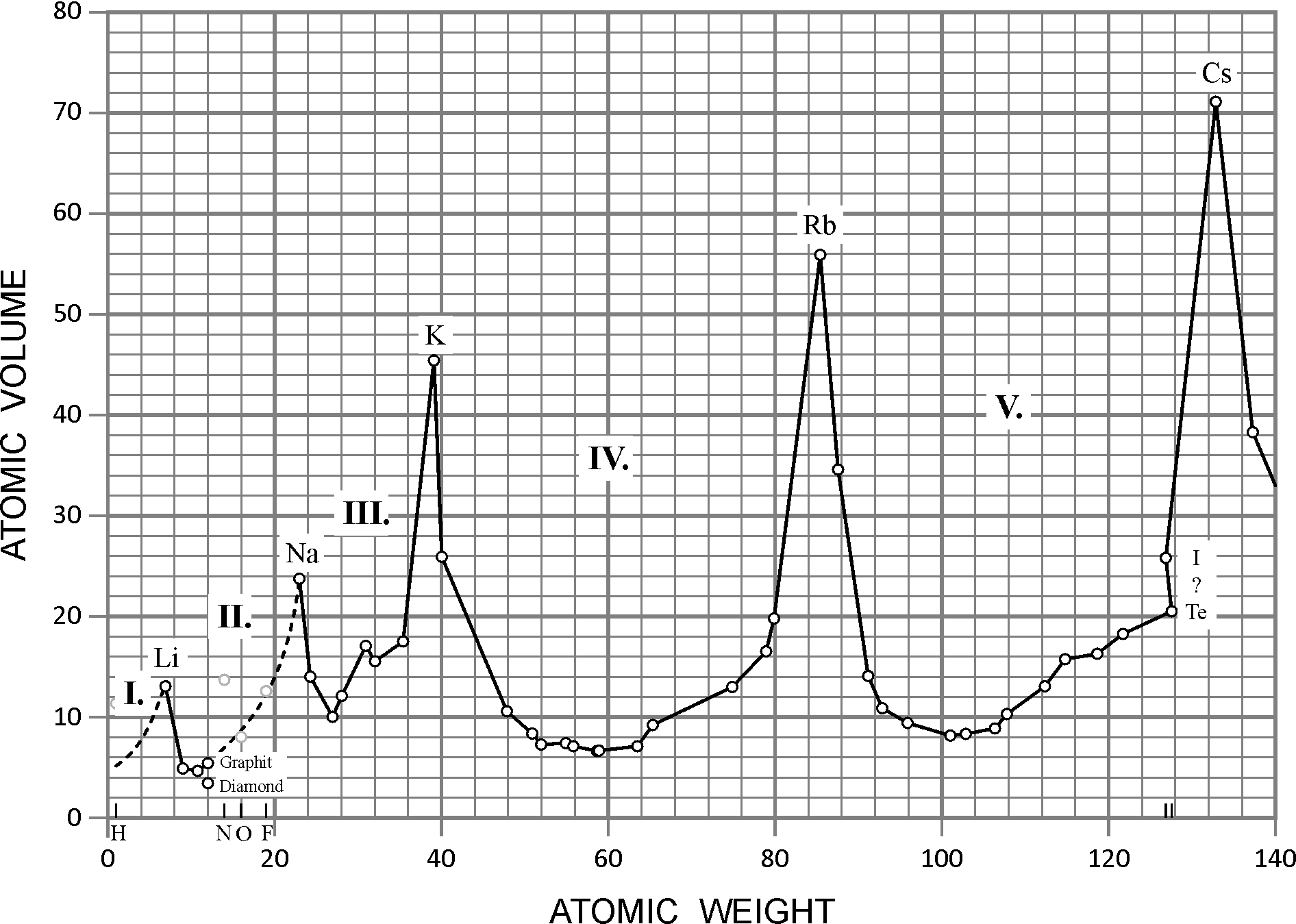
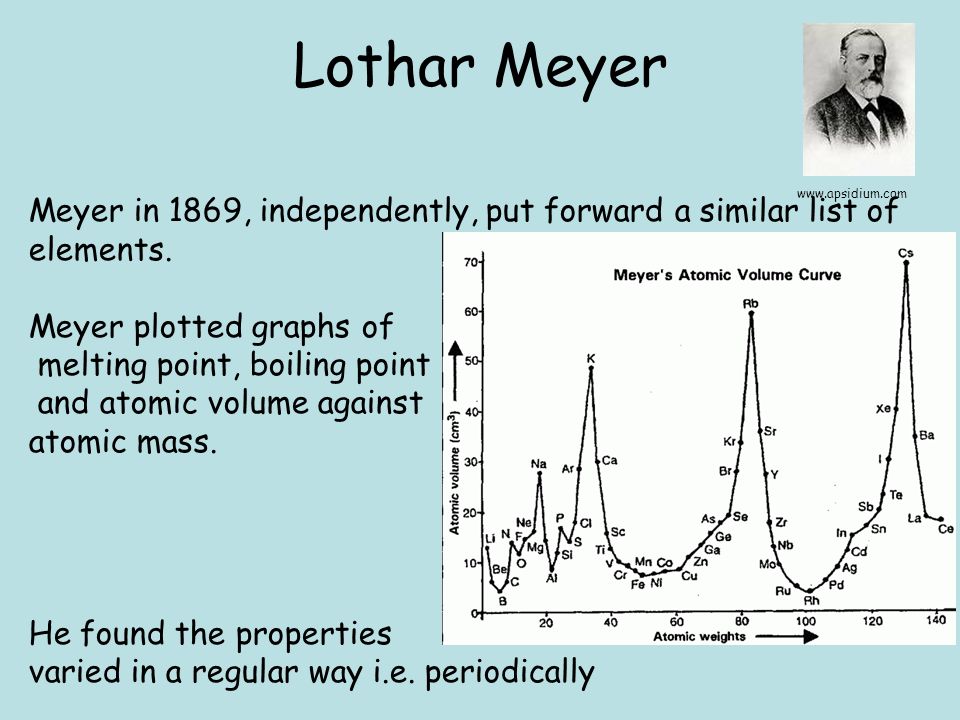
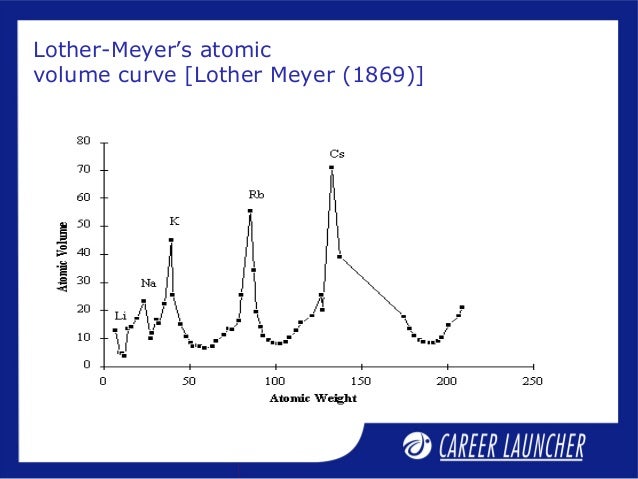
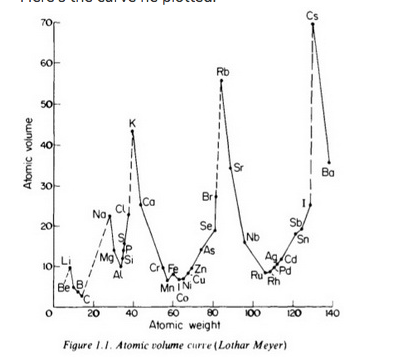
He arranged the elements in the order of their atomic weights they fall into groups in which similar chemical and physical properties are repeated at periodic intervals and in particular he showed that if the atomic weights are plotted as ordinates and the atomic volumes as abscissae the curve obtained presents a series of maxima and minima the most electro positive elements appearing at the peaks of the curve in the order of their atomic weights.

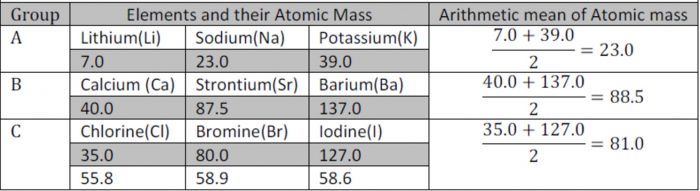
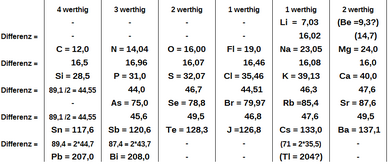
Lothar meyers classification in the form of curve. Classification of elements and periodicity in properties. Lothar meyers curve target audience. Geeth27 meritnation expert added an answer on 311016 lothar meyer classified elements not by atomic weight but by valence. B the alkaline earth metals be mg ca sr and ba and the elements forming basic oxides occupy the descending position on the curve chemist lothar meyer 1830 1895.
Lesson 2. Though originally educated as a physician he was chiefly interested in chemistry and physics. Julius lothar meyer 19 august 1830 11 april 1895 was a german chemisthe was one of the pioneers in developing the first periodic table of chemical elementsboth mendeleev and meyer worked with robert bunsenhe never used his first given name and was known throughout his life simply as lothar meyer. Class 11 unit 3.
19 1830 varel oldenburg germanydied april 11 1895 tuebingen german chemist who independently of dmitry mendeleyev developed a periodic classification of the chemical elements. Class 11th board exams neet jee advance. Lothar meyers atomic volume curve in 1869 a german chemist julius lothar meyer calculated atomic volumes of elements by dividing atomic mass of the element by its density. In 1859 meyer began his career as a science educator holding various appointments.
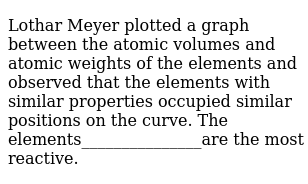
In these graphs he observed that element with similar physical properties occupy similar positions in the curve. How to grow the best tomatoes gardening tips and tricks duration.