Lothar Meyer Curve Depends On
Which of the following statement is wrong about lothar meyers plot between atomic volume against atomic weight.
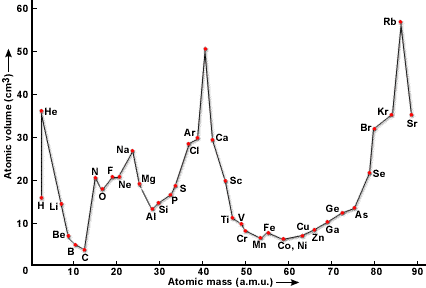
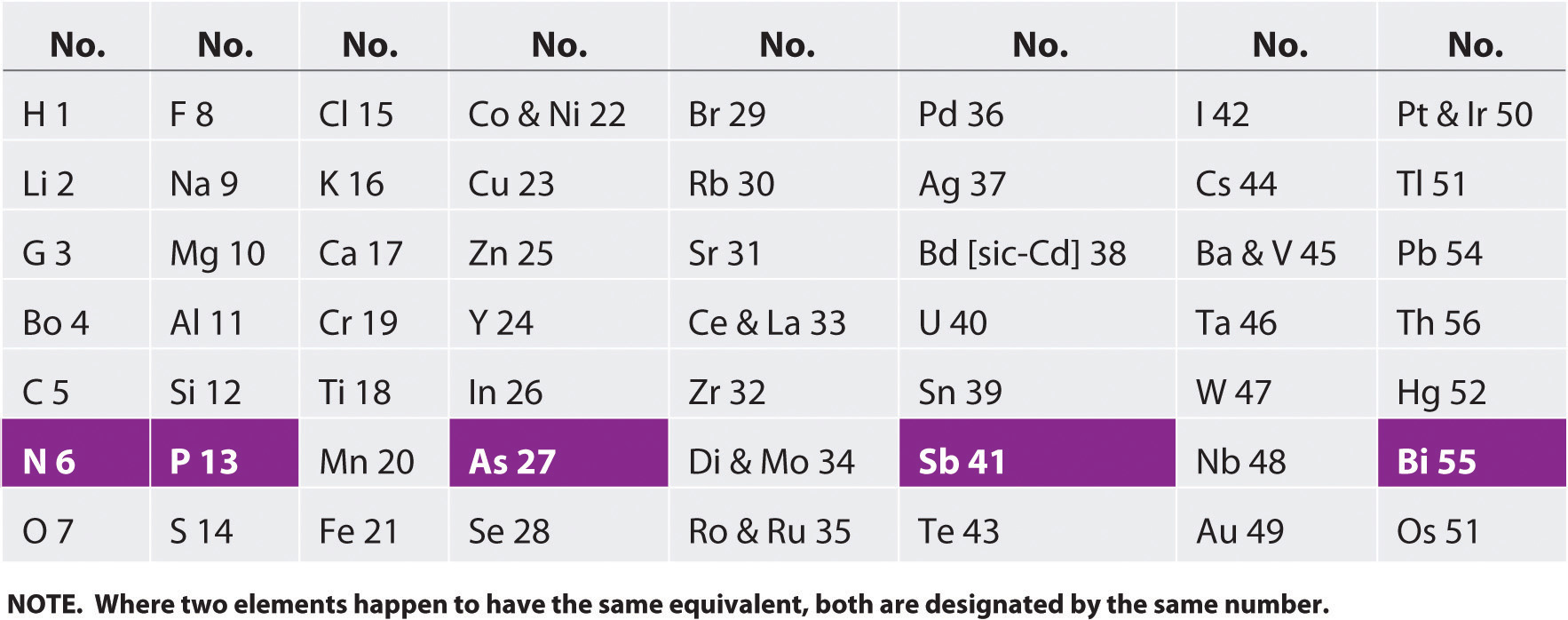
Lothar meyer curve depends on. Atomic weight in solid statedensity and he find out that elements with similar properties occupied the similar positions on the graph. On august 19 1830 german chemist julius lothar meyer was born. Julius lothar meyer was born on august 19 1830. Julius lothar meyer 19 august 1830 11 april 1895 was a german chemisthe was one of the pioneers in developing the first periodic table of chemical elementsboth mendeleev and meyer worked with robert bunsenhe never used his first given name and was known throughout his life simply as lothar meyer.
In 1859 meyer began his career as a science educator holding various lothar meyer german chemist who independently of dmitry mendeleyev developed a periodic classification of the chemical elements. He never used his first given name and was known throughout his life simply as lothar meyer. He was a german chemist but before he was a chemist he was a teacher at breslau karlsruheand tubingen. Elements having similar properties will occupy the same position in the curve.
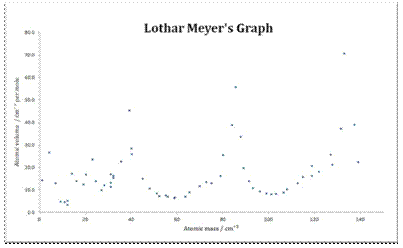
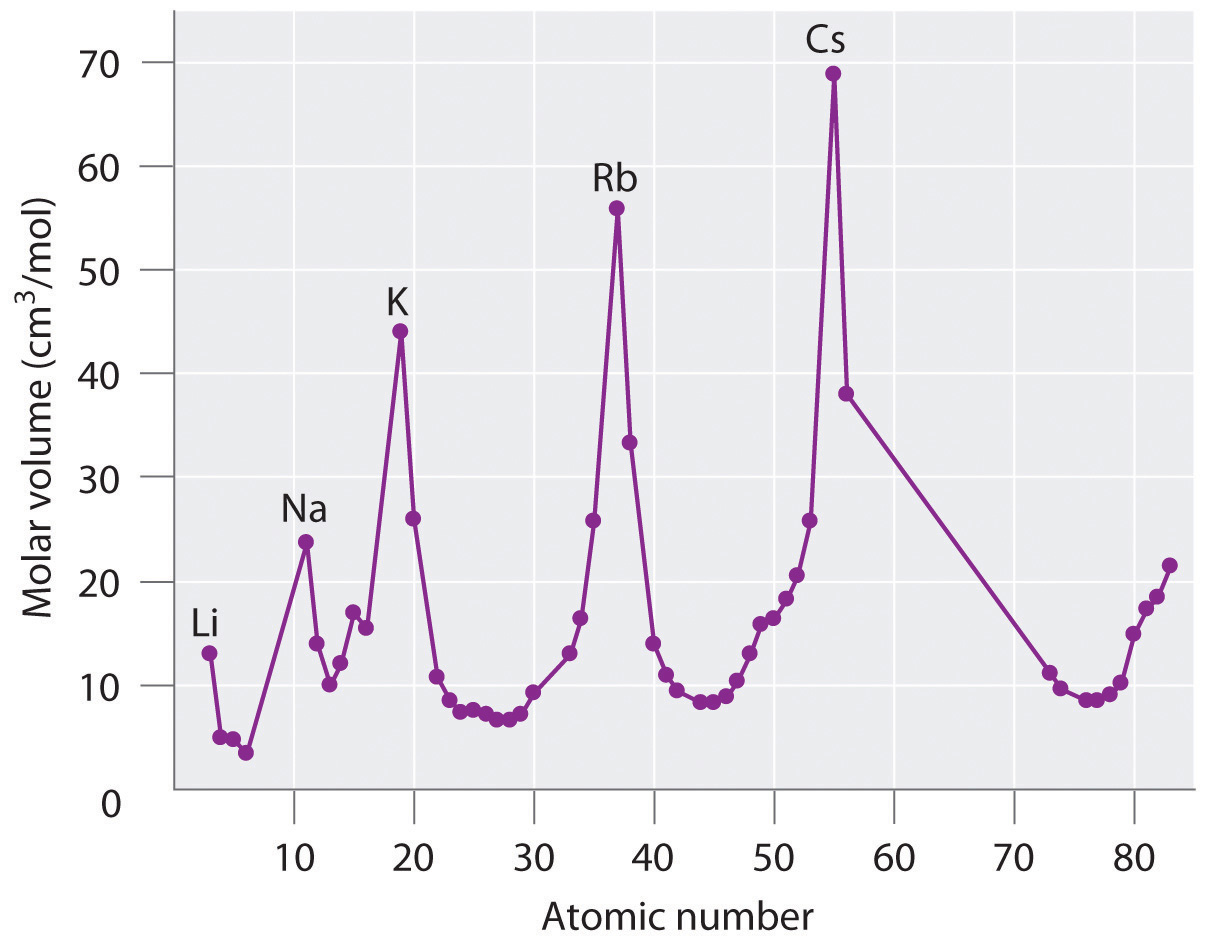
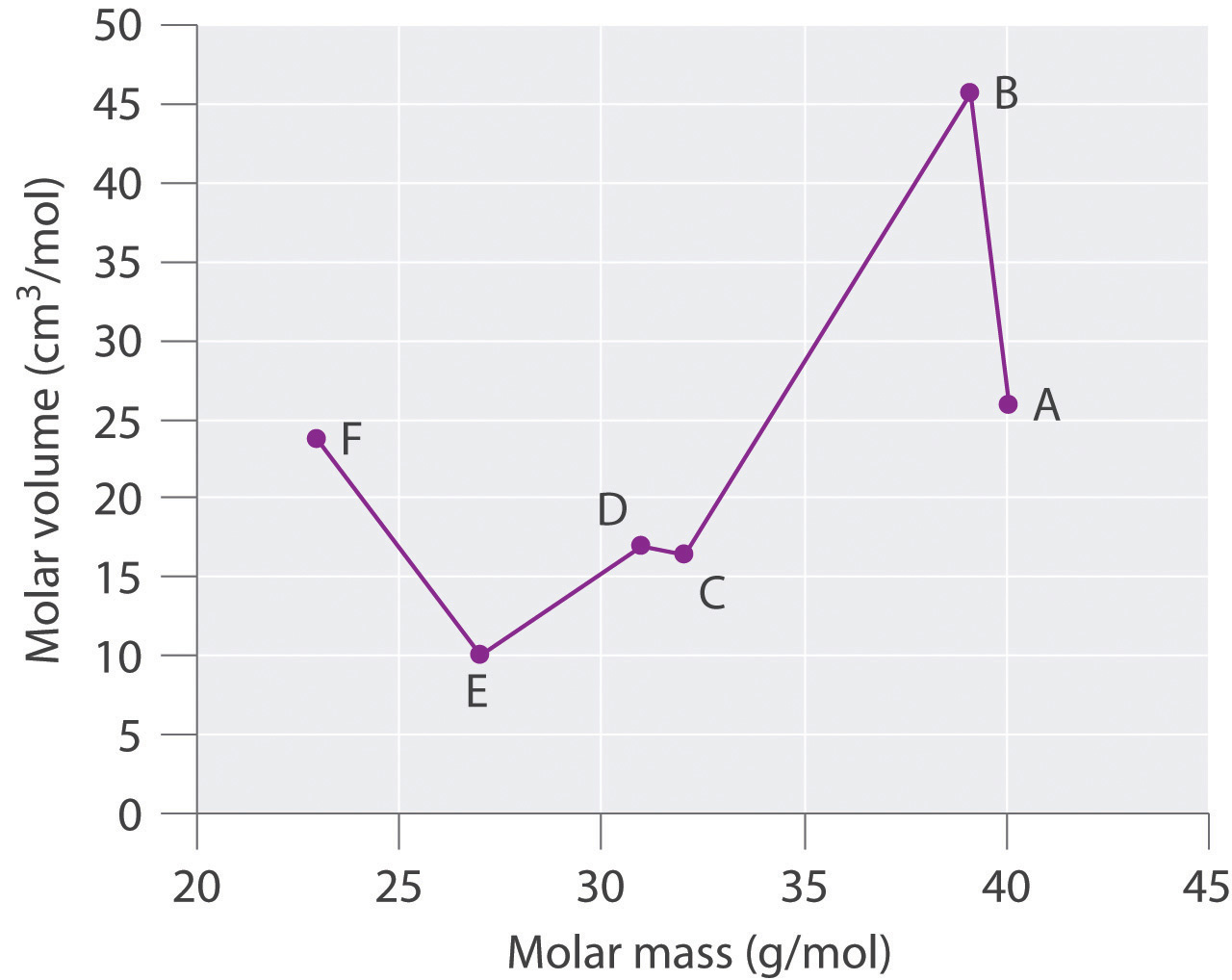
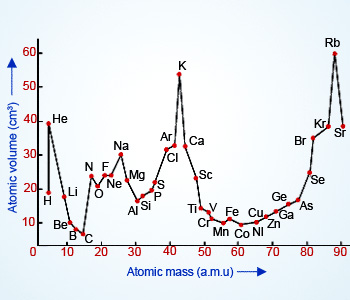
Sodium potassium rubidium and cesium. Lothar meyer meyers curve. Plotted the atomic volume of the elements against the atomic weight and found that the chemical properties of the element recur periodically. Each fall and rise to a peak corresponded to a period like the waves.
The peaks had alkali metals. Julius lothar meyer 19 august 1830 11 april 1895 was a german chemist. 1 alkali metal occupy peaks on the curve2 halogen atom occupy ascending positions on the curve3 alkaline earth metals occupy descending position on the curve4he proposed that physical properties of the element are a periodic function of their. Lothar meyer a german chemist plotted a graph between atomic weight and atomic volume ie.
If the atomic volumes of the elements were plotted against the atomic weight a series of peaks were produced. Lothar meyers atomic volume curve in 1869 a german chemist julius lothar meyer calculated atomic volumes of elements by dividing atomic mass of the element by its density. Meyer was into the volume curve in 1869 which represented the relation between the atomic weights and atomic volumes of the elements. Meyer was one of the pioneers in developing the first periodic table of chemical elementshe discovered the periodic law independently of dmitry mendeleev at about the same time 1869however he did not develop the periodic classification of the chemical elements as thoroughly as mendeleev.
Thus lothar meyer could determine the atomic volumes of elements.